
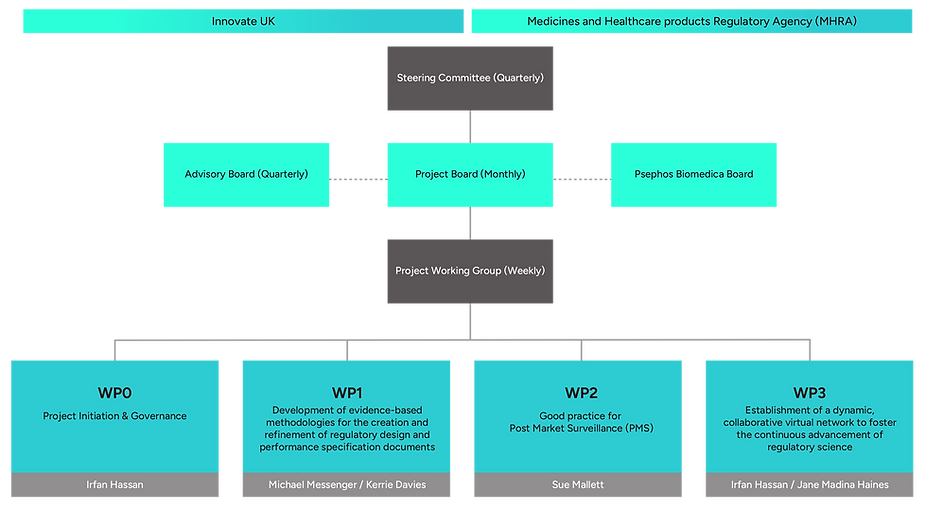
ORGANISATION STRUCTURE
PROJECT GOVERNANCE & OVERSIGHT
The CLEARED CERSI IVD Network is managed through a structured governance framework that ensures transparency, accountability, and effective delivery.
Regular meetings of the Project Board, Steering Committee, and Advisory Board provide continuous oversight, proactive risk management, and evidence-based decision-making. Robust communication strategies keep all partners and stakeholders aligned, while detailed reporting ensures full compliance with Innovate UK monitoring requirements.
Our Advisory Board, comprising experts from academia, industry, clinical end-users, patient representatives, and regulatory bodies, provides independent guidance and objective insight. Stakeholder feedback is integrated to drive continuous improvement and ensure the successful delivery and long-term impact of the CLEARED CERSI programme.
CLEARED (CLinical Evaluation & Assessment for REgulation of Diagnostic tests) Implementation Phase
OTHER PARTNERS

British In Vitro Diagnostics Association (BIVDA)
The British In Vitro Diagnostics Association (BIVDA), the UK’s leading trade association representing the IVD industry, is a key supporter of the CLEARED CERSI IVD Network. Representing companies from global organisations to innovative SMEs, BIVDA advocates for an evidence-based regulatory environment that supports safe, effective, and equitable diagnostic innovation.
Through its collaboration with CLEARED CERSI & BIVDA this partnership strengthens regulatory preparedness, fosters innovation, and advances the UK’s position as a global leader in diagnostic science and policy.